Comparison of classical cobalt containing pigments and alternatives from the 21st century
Many watercolours and other painting mediums are sold with identical or nearly identical pigment names, even though they don't necessarily contain those specific pigments. The purpose of this thesis is to examine the properties of the classical pigments aureolin, cobalt blue, cobalt violet and cerulean blue, and pigments that are sold with these names.
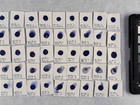
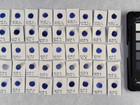
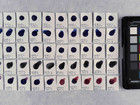
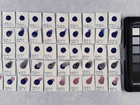
The first part of the experiments identifies the pigments in 16 purchased watercolours. This identification is performed using elemental analysis, which is carried out with SEM-EDX; afterwards, the results are used to make suggestions for the composition of pigments in the samples.
From the pigments identified with SEM-EDX, some were chosen to be compared to their classical counterparts; both physical and chemical properties are compared.
Benzimidazolone yellow is compared with aureolin, PB74 with cobalt blue, PV62 with cobalt violet and copper phthalocyanine blue is compared with cerulean blue. The comparison is carried out by making watercolour swatches with these pigments, the swatches are then examined under alkaline, sulfuric, weakly acidic and strongly acidic conditions, as well as in visible light.
CIE-L*a*b* colour measurements are made before and after the experiments, and the differences are used to compare the optical changes and the stability of the pigments.
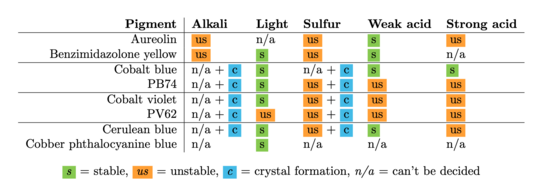
The results show that especially the pigments PV62 and PB74 are unstable in almost all the environments, and none of the pigments are clearly stable in all the environments tested in this thesis.
Identification of pigments in watercolours samples
Before comparing properties of the pigments, it was necessary to identify the pigments to be compared. The identification was done with elemental analysis carried out by SEM-EDX. By doing the analysis with SEM-EDX it is possible to get the elemental composition of a sample with w/w% of the different elements.
By comparing the pigment informations from the manufacturer with the measured values it was possible to make suggestions for the composition of pigments in the specific sample.
It wasn't possible to find any information on the ingredients of the binding media in the samples, therefore this was assumed to be of pure gum arabic, after the chemical formula, (C5H8O4)3(C14H23O10)2.
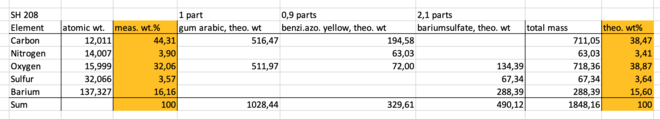
An example of determining the pigment composition of sample 'SH 208' is shown below. SH 208 is sold with the name 'Aureolin modern', but is known to contain the pigment benzimidazolone yellow, C18H18N5O5. Beside the elements in the pigment, both barium and sulfur were detected in the analysis, with may come from the common filler bariumsulfate, BaSO4.
Note that hydrogen is not included in the calculations, as it isn't possible to detect any elements lighter that boron using SEM-EDX.
The calculations show, that the wt% from the analysis may fit, if the sample contain 0,9 parts benzimidazolone yellow and 2,1 parts bariumsulfate per 1 part gum arabic.
From the identifications, four pigments were chosen and their physical and chemical properties were examined.
Benzimidazolone yellow is to be compared with aureolin.
PB74 is compared to cobalt blue.
PV62 is compared to cobalt violet.
Cobber phthalocyanine blue is compared to cerulean blue.
Examination of physical and chemical properties
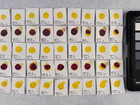
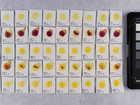
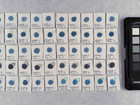
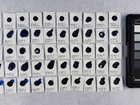
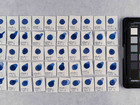
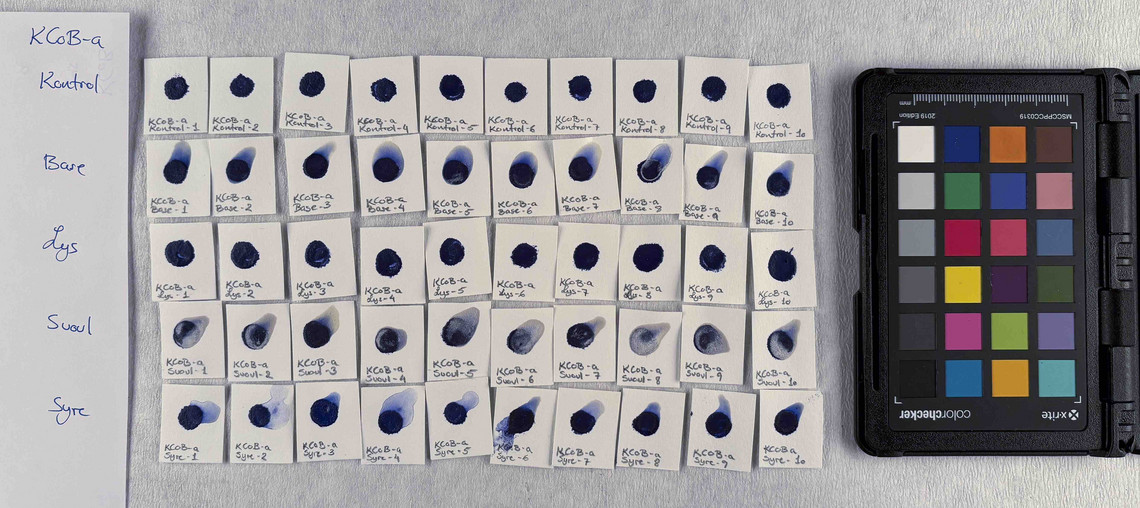
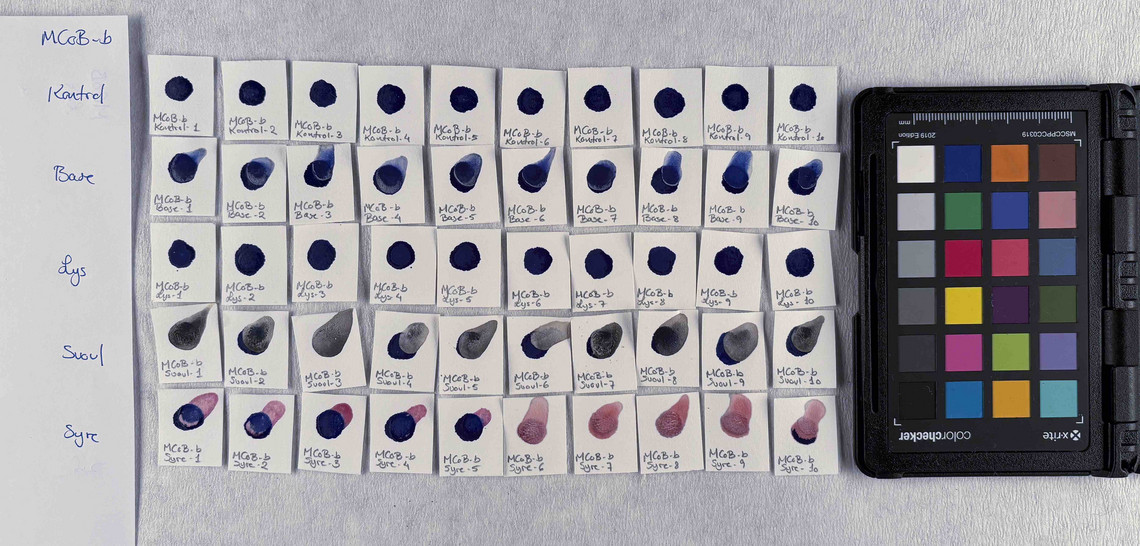
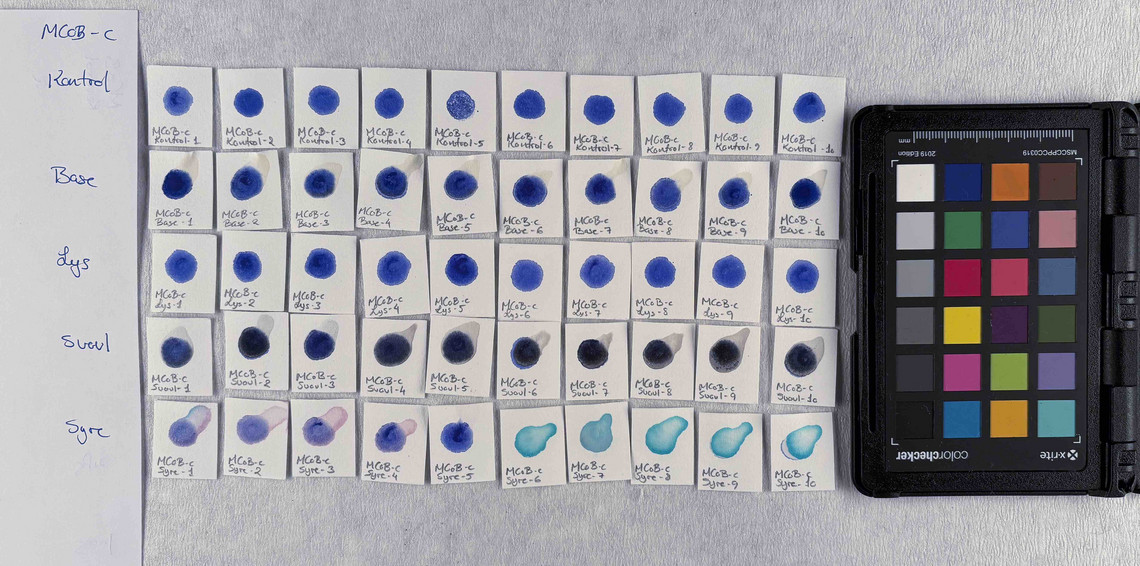
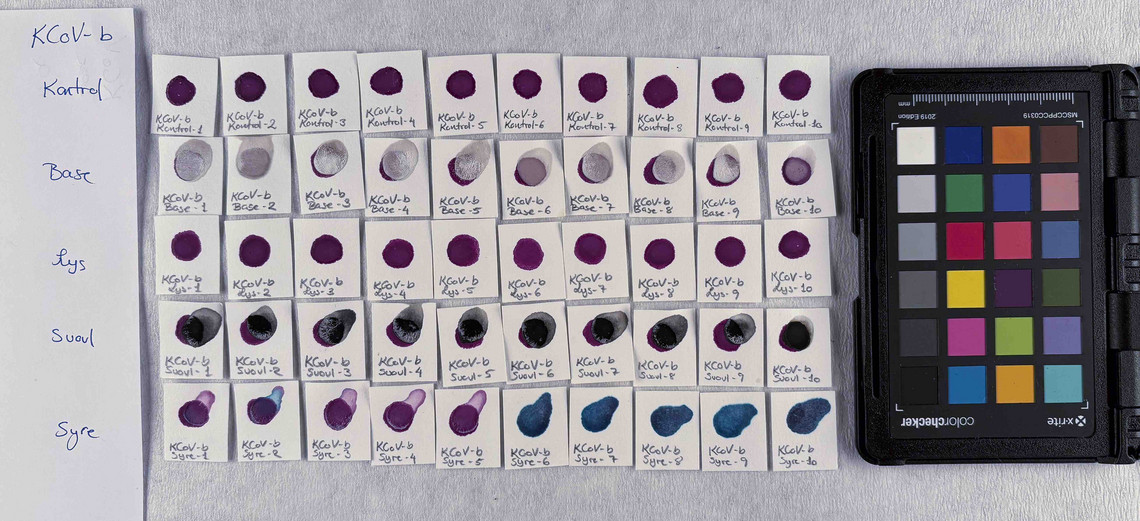
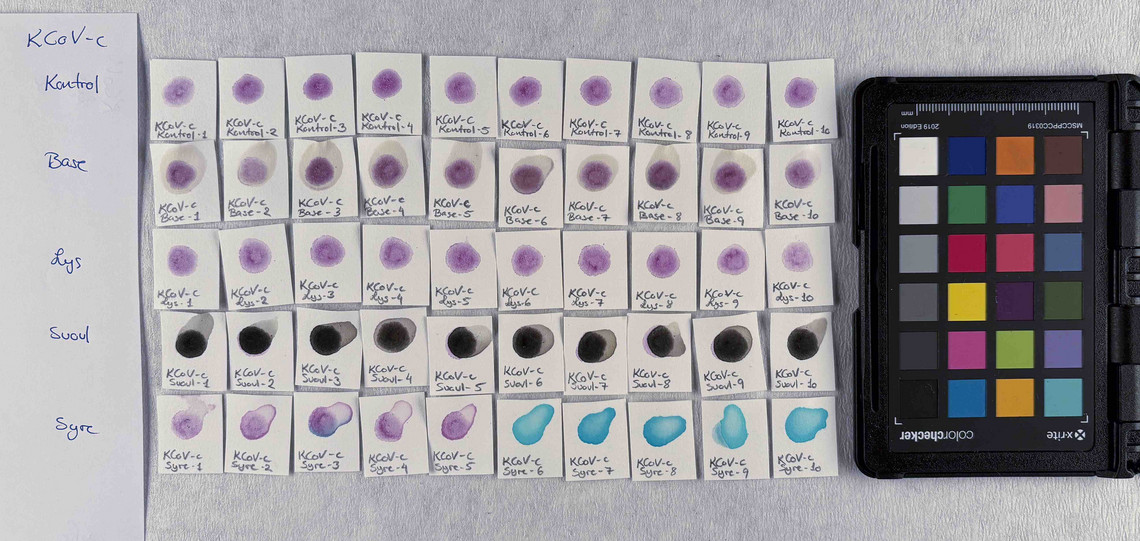
Samples of watercolour are made with the identified pigments as well as their classical counterparts in three different concentrations in water.
Conc. a = pure watercolour
Conc. b = 50%
Conc. c = 5%
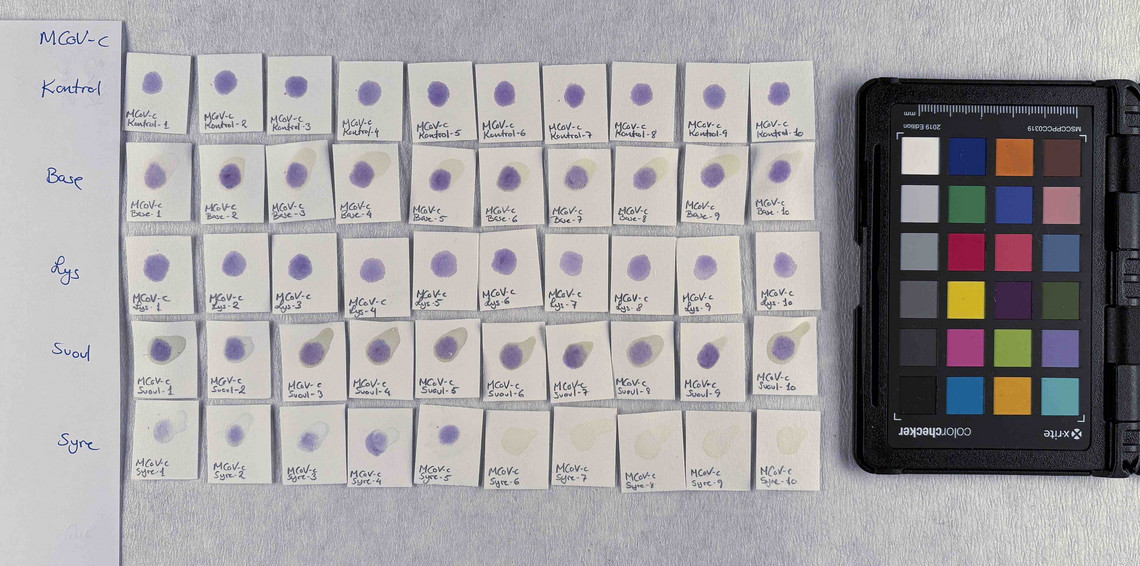
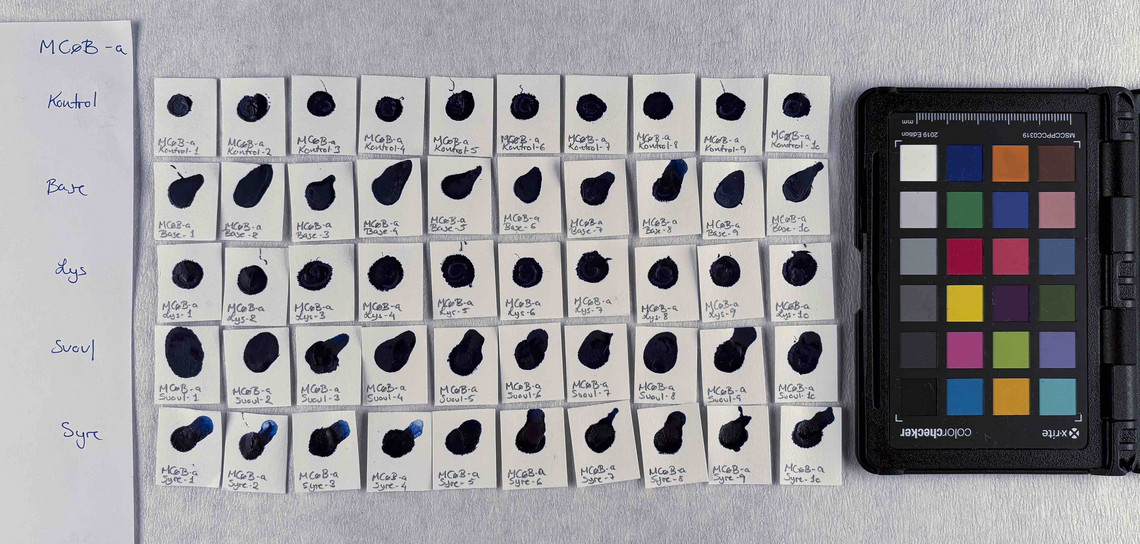
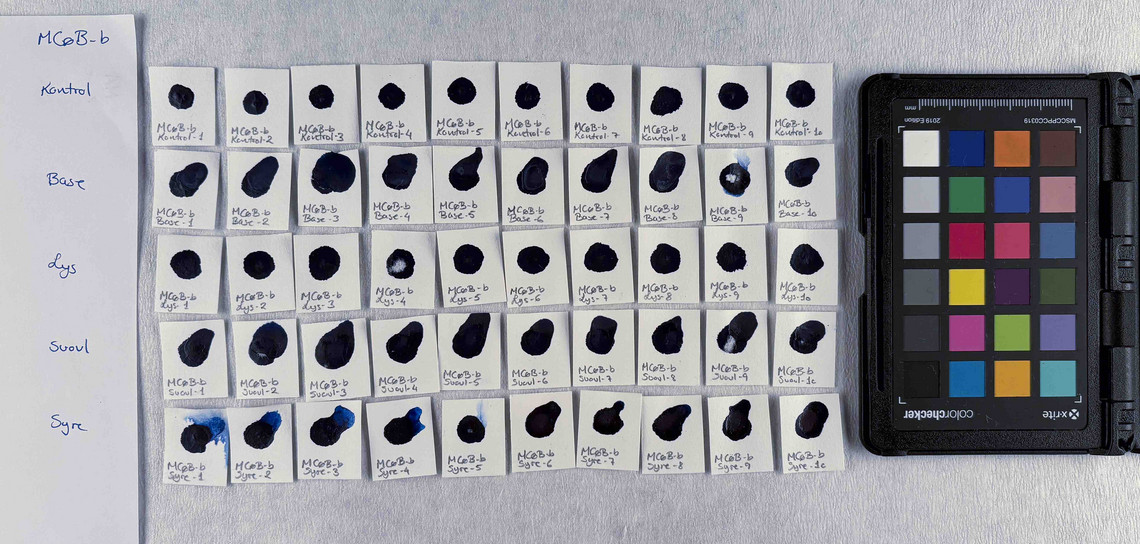
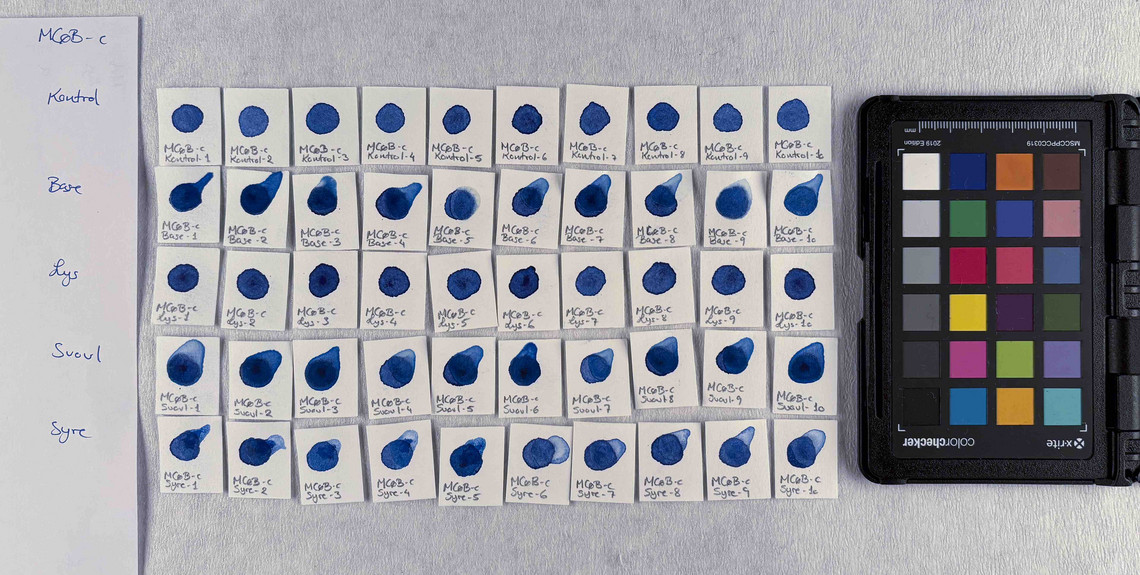
The pigments stability are examined under alkaline, sulfuric, weakly acidic and strongly acidic conditions, using respectively 2 M sodium hydroxide, NaOH, 1 M sodium sulfide, Na2S, 4 M acetic acid, CH3COOH, and 4 M hydrochloric acid, HCl. In addition they are examined in visible light using microfading.
The stability is evaluated visually and by comparing CIE-L*a*b* colour measurements from before and after the treatment.
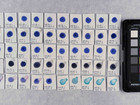
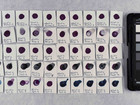
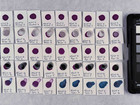
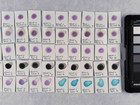
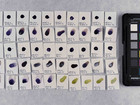
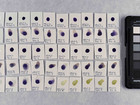
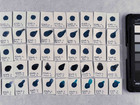
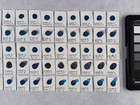
Photo documentation of the pigment samples after treatment:
The results are summarised below. They do not point out one pigment as the best, but they provide information on how to treat the different pigments, in both active and preventive conservation.
Many pigments may look alike, but are chemically very different. Many of the samples from the elemental analyses didn't contain the pigment, after which they were named. With a bit of research it was however, (often on the manufacturers website) possible to find pigment codes for the samples. The pigment codes can be connected to a chemical formula for the pigment in the sample and in all the analysed samples, the information from the pigment codes matched the measured data partly or completely.
Even though the pigment name on the watercolour samples don't match the content, it is possible to find information on the pigments in a sample.
The pigment chosen for a task should be selected based on the desired properties and the object of which it concerns.